Your Computer software assurance guidance fda images are ready in this website. Computer software assurance guidance fda are a topic that is being searched for and liked by netizens now. You can Find and Download the Computer software assurance guidance fda files here. Get all royalty-free photos.
If you’re looking for computer software assurance guidance fda pictures information connected with to the computer software assurance guidance fda keyword, you have visit the right site. Our website always gives you suggestions for seeking the highest quality video and picture content, please kindly hunt and locate more informative video content and images that match your interests.
Computer Software Assurance Guidance Fda. As part of on-going regulation development and improvement the FDA via the CDRH Center for Devices and Radiological Health the CBER Center for Biologics Evaluation and Research and the CDER Center for Drug Evaluation and Research are developing new software assurance guidance the Computer Software Assurance for Manufacturing Operations and Quality. The FDAs guidance document titled Computer Software Assurance for Manufacturing and Quality System Software has its roots in a 2011 FDA study of the Case for Quality which examined the 2002 guidance document called Validation of Software in Medical Devices. FDAs Upcoming Computer Software Assurance Guidance Regulatory Consulting Kathleen Warner PhD RCM Technologies wrote in the current issue of meddeviceonline. The FDA is expected to release its new guidance around CSA Computer Software Assurance for Manufacturing Operations and Quality Systems Software before the end of 2020.
 Computer Software Assurance Csa The New Csv From 3-14.com
Computer Software Assurance Csa The New Csv From 3-14.com
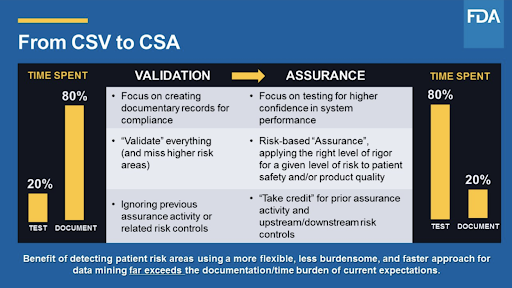
The Guidance is on FDAs list for release in September 2020 and applies to non-product quality system software solutions. The new draft guidance will apply critical thinking assessment and risk tools for assuring that data for the manufacture of. Computer Software Assurance CSA Computer system validation CSV is a much more familiar term than CSA but CSA is the wave of the future in validating your software. The FDA realizing this is counterproductive initiated the CSA Computer Software Assurance guidelines so that 80 of the manufacturers time is spent on critical thinking and testing and only 20 of their time in documentation. New Draft Guidance to Support Risk-Based Computer Software Assurance. FDA Computer Software Assurance guidance release date This new draft guidance to support risk-based computer software assurance is in the FDAs A-List of guidance documents that the Agency intends to publish during FY2020 ie.
FDAs Upcoming Computer Software Assurance Guidance Regulatory Consulting Kathleen Warner PhD RCM Technologies wrote in the current issue of meddeviceonline.
Computer Software Assurance CSA is a Case for Quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization and innovation. The FDAs general view of automation is basically a green light for companies. The next step in the Computer Software Assurance process is to determine a risk-based approach focusing in on safety and quality specifically high-risk areas that may require the most rigorous assurance effort and objective evidence which is complete extensive validation and ensure the high-risk features functions or operations reliably perform as intended. The FDAs New Approach to CSV Jul 15 2021 Testing Validation During a 2011 review of medical device quality data the FDAs Center for Devices and Radiological Health CDRH noticed a variety of widespread manufacturing risks that were impacting product quality. The FDA realizing this is counterproductive initiated the CSA Computer Software Assurance guidelines so that 80 of the manufacturers time is spent on critical thinking and testing and only 20 of their time in documentation. Before September 30th 2020.
 Source: americanpharmaceuticalreview.com
Source: americanpharmaceuticalreview.com
As the FDA finalizes and publishes the Computer Software Assurance CSA guidance later this year companies that have not already started the transformation process to CSA can get started. The FDA outlined a new. As the FDA finalizes and publishes the Computer Software Assurance CSA guidance later this year companies that have not already started the transformation process to CSA can get started. What is the FDAs new guidance for computer software assurance. The FDAs New Approach to CSV Jul 15 2021 Testing Validation During a 2011 review of medical device quality data the FDAs Center for Devices and Radiological Health CDRH noticed a variety of widespread manufacturing risks that were impacting product quality.
 Source: criticalmanufacturing.com
Source: criticalmanufacturing.com
FDA Computer Software Assurance guidance release date This new draft guidance to support risk-based computer software assurance is in the FDAs A-List of guidance documents that the Agency intends to publish during FY2020 ie. Before September 30th 2020. As the FDA finalizes and publishes the Computer Software Assurance CSA guidance later this year companies that have not already started the transformation process to CSA can get started. The FDA draft guidance on Computer Software Assurance is a paradigm shift from document focused computer system validation to critical thinking assurance practices. The FDAs guidance document titled Computer Software Assurance for Manufacturing and Quality System Software has its roots in a 2011 FDA study of the Case for Quality which examined the 2002 guidance document called Validation of Software in Medical Devices.
 Source: amplelogic.com
Source: amplelogic.com
Applied to any software. Draft Guidance Topics. As the FDA finalizes and publishes the Computer Software Assurance CSA guidance later this year companies that have not already started the transformation process to CSA can get started. The FDA is expected to release a new guidance document Computer Software Assurance for Manufacturing Operations and Quality System Software in 2021. Computer Software Assurance CSA is a Case for Quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization and innovation.
 Source: perfval.com
Source: perfval.com
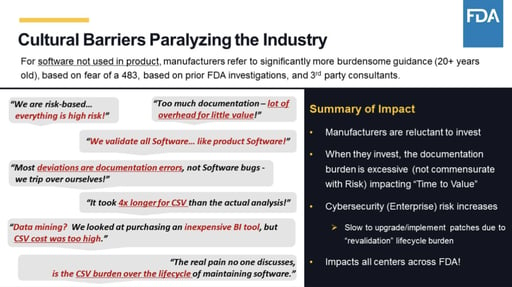
FDA continues to encourage the use of innovative new technologies to support the development of quality new drugs and ensure that patient safety is uppermost in the development and manufacture of drugs. The FDA realizing this is counterproductive initiated the CSA Computer Software Assurance guidelines so that 80 of the manufacturers time is spent on critical thinking and testing and only 20 of their time in documentation. Draft Guidance Topics. Ritchie MS Senior Validation Engineer Consultant PSC Biotech Corporation. FDA Activities and Engagement with the Voluntary Improvement.
 Source: slcontrols.com
Source: slcontrols.com
Fostering Medical Device Improvement. Computer Software Assurance for Production and Quality System Software. The FDA recognizes the value of using advanced technologies to enable the industry to make medicines of more reliable quality. This new guidance is highly anticipated because it will actually streamline some of your computer software systems. Data Integrity by Design.
 Source: techsolcorp.com
Source: techsolcorp.com
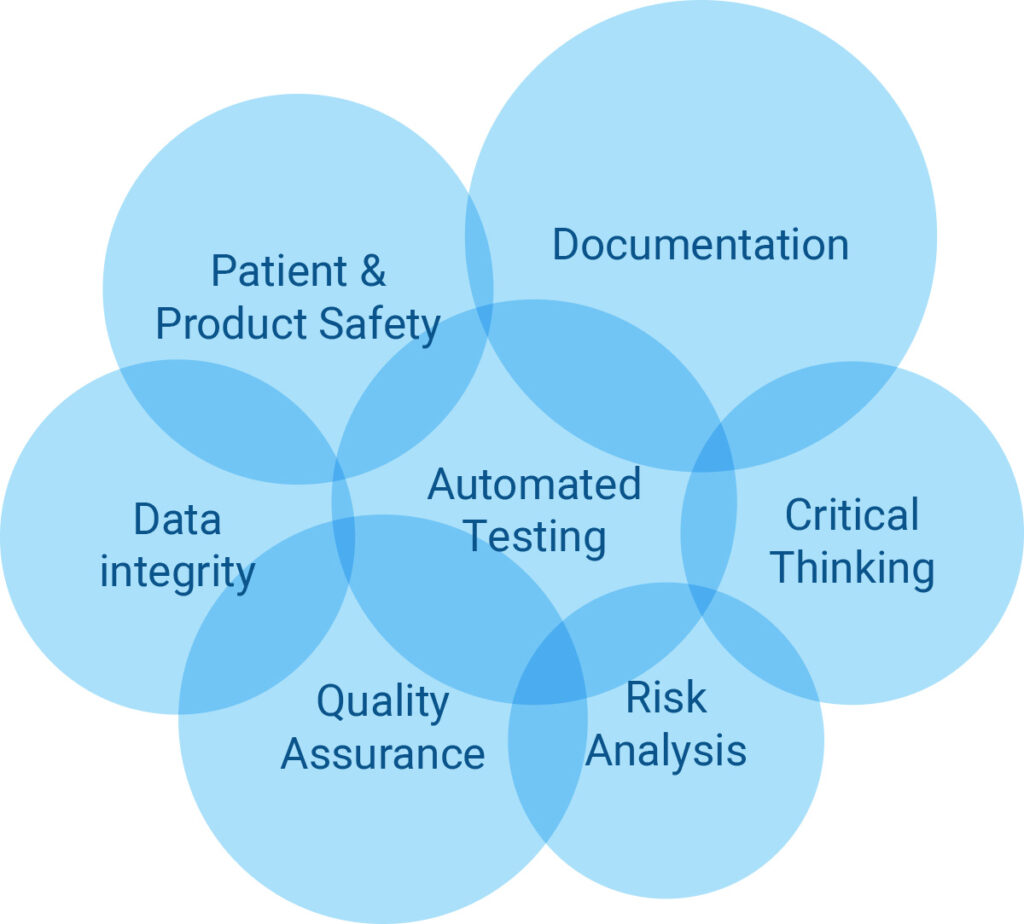
The FDAs guidance document titled Computer Software Assurance for Manufacturing and Quality System Software has its roots in a 2011 FDA study of the Case for Quality which examined the 2002 guidance document called Validation of Software in Medical Devices. The FDA realizing this is counterproductive initiated the CSA Computer Software Assurance guidelines so that 80 of the manufacturers time is spent on critical thinking and testing and only 20 of their time in documentation. New Draft Guidance to Support Risk-Based Computer Software Assurance. FDAs Upcoming Computer Software Assurance Guidance Regulatory Consulting Kathleen Warner PhD RCM Technologies wrote in the current issue of meddeviceonline. As per FDAs forthcoming guidance announcement the new Computer Software Assurance CSA process is primarily focused on identifying intended use of the medical device or software system checking its impact on patient product safety and quality identifying related risks applying critical thinking and assurance needs then finally execute testing and generate.
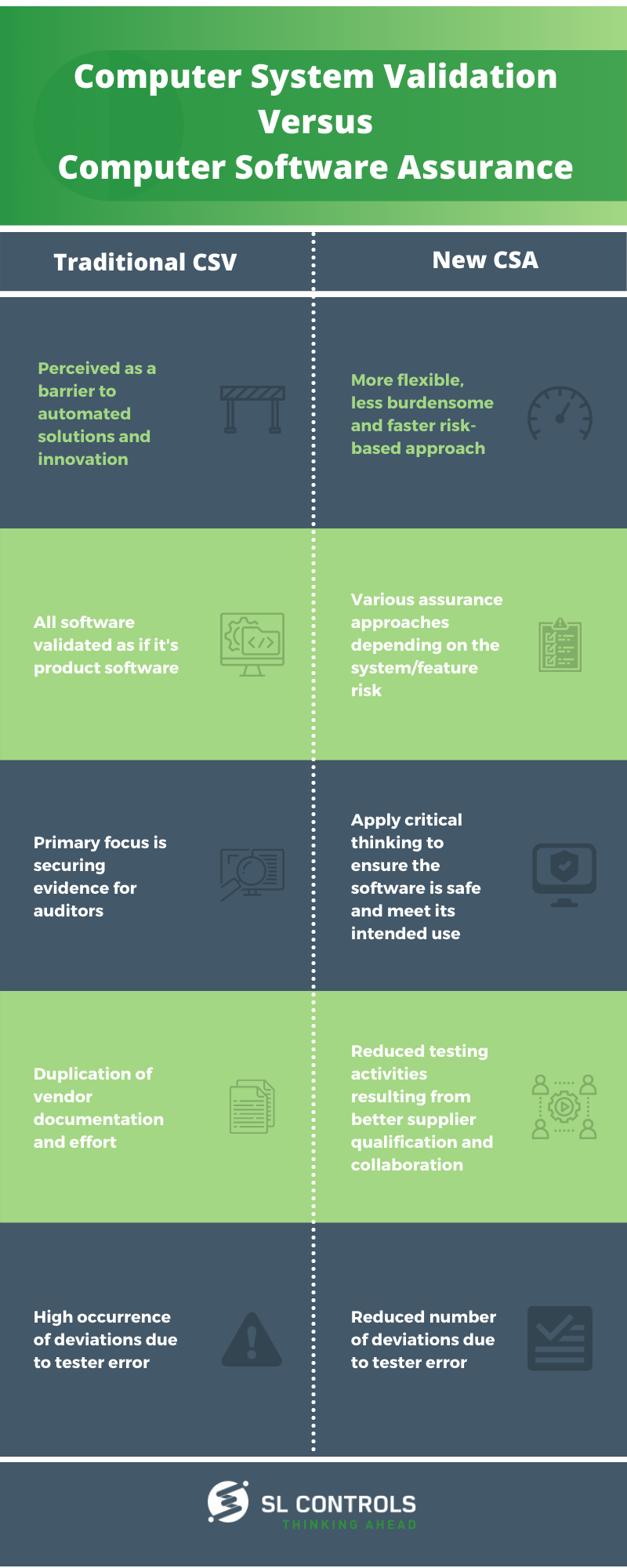 Source: slcontrols.com
Source: slcontrols.com
Computer Software Assurance CSA. The FDAs general view of automation is basically a green light for companies. The new FDA guidance will focus on non-product quality systems such as bug tracking document management and lifecycle management systems. This new guidance is highly anticipated because it will actually streamline some of your computer software systems. In 2019 FDA will be releasing a new draft guidance Computer Software Assurance for Manufacturing Operations and Quality System Software that updates 20 year legacy guidance documents found in 21 CFR Part 11 relating to medical device computer system validation and software validation.
 Source: 3-14.com
Source: 3-14.com
Drafting Up the Computer Software Assurance CSA Set for release in the fiscal year 2020 the FDA has drafted up a guidance to Computer Software Assurance for Manufacturing Operations and Quality System Software to address the pain points in the following ways. FDAs Upcoming Computer Software Assurance Guidance Regulatory Consulting Kathleen Warner PhD RCM Technologies wrote in the current issue of meddeviceonline. The FDA draft guidance on Computer Software Assurance is a paradigm shift from document focused computer system validation to critical thinking assurance practices. Fostering Medical Device Improvement. And the impact this will have on current and future computerised system implementations.
 Source: kalleid.com
Source: kalleid.com
Computer Software Assurance CSA Computer system validation CSV is a much more familiar term than CSA but CSA is the wave of the future in validating your software. The FDA is leaning towards a Case for Quality CfQ approach with less emphasis on a compliance. The FDA recognizes the value of using advanced technologies to enable the industry to make medicines of more reliable quality. FDA Activities and Engagement with the Voluntary Improvement. For FDA purposes this guidance applies to any software related to a regulated medical device as defined by Section 201h of.
Source: greenlight.guru
The FDA will release the draft guidance on Computer Software Assurance for Manufacturing Operations and Quality System Software in late 2020. The FDA realizing this is counterproductive initiated the CSA Computer Software Assurance guidelines so that 80 of the manufacturers time is spent on critical thinking and testing and only 20 of their time in documentation. As per FDAs forthcoming guidance announcement the new Computer Software Assurance CSA process is primarily focused on identifying intended use of the medical device or software system checking its impact on patient product safety and quality identifying related risks applying critical thinking and assurance needs then finally execute testing and generate. Although not yet officially released people are starting to comment on what will be outlined in the guidance. Computer Software Assurance for Production and Quality System Software.
 Source: kenx.org
Source: kenx.org
As the FDA finalizes and publishes the Computer Software Assurance CSA guidance later this year companies that have not already started the transformation process to CSA can get started. Computer Software Assurance CSA is a Case for Quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization and innovation. FDA Activities and Engagement with the Voluntary Improvement. Computer Software Assurance for Production and Quality System Software. FDA Computer Software Assurance guidance release date This new draft guidance to support risk-based computer software assurance is in the FDAs A-List of guidance documents that the Agency intends to publish during FY2020 ie.
 Source: aodocs.com
Source: aodocs.com
As the FDA finalizes and publishes the Computer Software Assurance CSA guidance later this year companies that have not already started the transformation process to CSA can get started. As part of on-going regulation development and improvement the FDA via the CDRH Center for Devices and Radiological Health the CBER Center for Biologics Evaluation and Research and the CDER Center for Drug Evaluation and Research are developing new software assurance guidance the Computer Software Assurance for Manufacturing Operations and Quality. As per FDAs forthcoming guidance announcement the new Computer Software Assurance CSA process is primarily focused on identifying intended use of the medical device or software system checking its impact on patient product safety and quality identifying related risks applying critical thinking and assurance needs then finally execute testing and generate. Computer Software Assurance CSA is a Case for Quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization and innovation. Although not yet officially released people are starting to comment on what will be outlined in the guidance.
 Source: 3-14.com
Source: 3-14.com
Although not yet officially released people are starting to comment on what will be outlined in the guidance. What is the FDAs new guidance for computer software assurance. The new FDA guidance will focus on non-product quality systems such as bug tracking document management and lifecycle management systems. Computer Software Assurance CSA is a Case for Quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization and innovation. Applied to any software.
![]() Source: amplelogic.com
Source: amplelogic.com
This new guidance is highly anticipated because it will actually streamline some of your computer software systems. The FDA is expected to release a new guidance document Computer Software Assurance for Manufacturing Operations and Quality System Software in 2021. As part of on-going regulation development and improvement the FDA via the CDRH Center for Devices and Radiological Health the CBER Center for Biologics Evaluation and Research and the CDER Center for Drug Evaluation and Research are developing new software assurance guidance the Computer Software Assurance for Manufacturing Operations and Quality. The FDA is leaning towards a Case for Quality CfQ approach with less emphasis on a compliance. FDA Activities and Engagement with the Voluntary Improvement.
 Source: aodocs.com
Source: aodocs.com
Before September 30th 2020. The Guidance is on FDAs list for release in September 2020 and applies to non-product quality system software solutions. The next step in the Computer Software Assurance process is to determine a risk-based approach focusing in on safety and quality specifically high-risk areas that may require the most rigorous assurance effort and objective evidence which is complete extensive validation and ensure the high-risk features functions or operations reliably perform as intended. FDAs Upcoming Computer Software Assurance Guidance Regulatory Consulting Kathleen Warner PhD RCM Technologies wrote in the current issue of meddeviceonline. The new draft guidance will apply critical thinking assessment and risk tools for assuring that data for the manufacture of.
 Source: 3-14.com
Source: 3-14.com
As per FDAs forthcoming guidance announcement the new Computer Software Assurance CSA process is primarily focused on identifying intended use of the medical device or software system checking its impact on patient product safety and quality identifying related risks applying critical thinking and assurance needs then finally execute testing and generate. What is the FDAs new guidance for computer software assurance. The new draft guidance will apply critical thinking assessment and risk tools for assuring that data for the manufacture of. The next step in the Computer Software Assurance process is to determine a risk-based approach focusing in on safety and quality specifically high-risk areas that may require the most rigorous assurance effort and objective evidence which is complete extensive validation and ensure the high-risk features functions or operations reliably perform as intended. The FDA specifically uses the language any software that is not directly used in a medical device medical device as a service or end.
 Source: criticalmanufacturing.com
Source: criticalmanufacturing.com
FDA Activities and Engagement with the Voluntary Improvement. The Guidance is on FDAs list for release in September 2020 and applies to non-product quality system software solutions. In an industry first members of the Computer Software Assurance CSA team responsible for working with the FDA to develop the draft guidance on this topic have collaborated closely with ISPE GAMP subject matter experts to create an appendix to the ISPE GAMP RDI Good Practice Guide. Computer Software Assurance CSA is a Case for Quality initiative that arose when computer systems validation was identified as a barrier to adopting technology for modernization and innovation. In 2019 FDA will be releasing a new draft guidance Computer Software Assurance for Manufacturing Operations and Quality System Software that updates 20 year legacy guidance documents found in 21 CFR Part 11 relating to medical device computer system validation and software validation.
 Source: slcontrols.com
Source: slcontrols.com
Ritchie MS Senior Validation Engineer Consultant PSC Biotech Corporation. What is the FDAs new guidance for computer software assurance. Data Integrity by Design. FDA continues to encourage the use of innovative new technologies to support the development of quality new drugs and ensure that patient safety is uppermost in the development and manufacture of drugs. The FDA outlined a new.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title computer software assurance guidance fda by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







